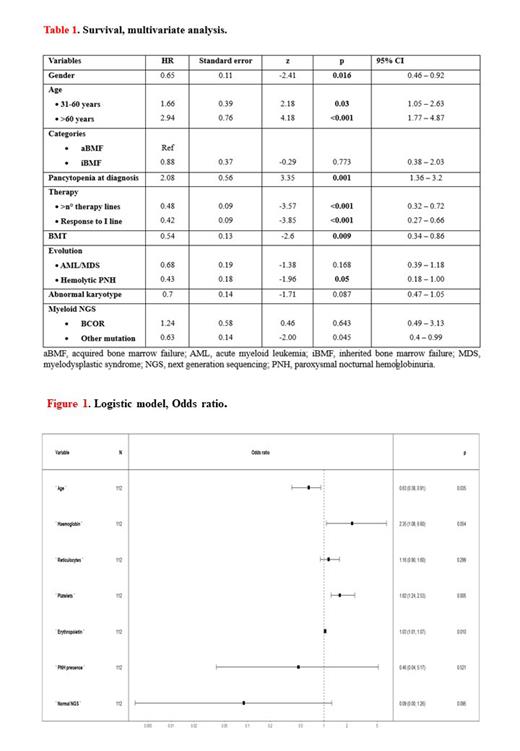
The wide overlaps in clinical and hematologic features of inherited (iBMF) and acquired (aBMF) bone marrow failures are often the cause of diagnostic difficulties and delay in proper therapeutic approaches. Screening tests for iBMF are complex and expensive and have limited availability. Data-driven machine learning algorithms may reveal useful tools in clinical practice to guide differential diagnosis. We collected data about clinical, laboratory and natural history of 597 patients with BMF (55 iBMF; 542 aBMF). iBMFs were typically younger (30 y, 0.1-71 vs 44 y, 2-85, p <0.001) and presented higher baseline blood counts (reticulocytes 62x10^9/L, 31-205 vs 35x10^9/L, 0-216; neutrophiles 1.4x10^9/L, 0-4.3 vs 0.6x10^9/L, 0-4.6; platelets 93x10^9/L, 7-356 vs 23x10^9/L, 1-355, p <0.001), and more cytogenetic aberrations (32% vs 13%, p 0.002). Conversely, PNH clones were detected and confirmed overtime mainly in aBMF (51% vs 9% p <0.001), with median clone size of 0.6% (range 0.02-3.8) in iBMF. By NGS analysis, no differences in acquired myeloid gene mutations incidence were detected between the groups (25% vs 13%, p 0.1). Evolution to MDS occurred in 20% of iBMF vs 11% of aBMF (p 0.04), and to AML in 3.6% vs 1.5% (p 0.2). Time between diagnosis and evolution was shorter in iBMF (2 y, 0-14 vs 5.4 y, 0.2-32 p 0.01). Survival was similar between the 2 groups with median values of 7 y (95%CI, 6.1-7.6), reaching 8 y (95%CI, 6.9-9.2) in transplanted patients. Factors significantly associated with a better survival were female gender (HR: 0.73, 95%CI: 0.59-0.90, p 0.004), BMT (HR: 0.62, 95%CI: 0.47-0.83, p 0.001), number of therapy lines (HR: 0.67, 95%CI: 0.51-0.87, p 0.003), response to 1 st line (HR: 0.53, 95%CI: 0.41-0.69, p < 0.001), and evolution to PNH (HR: 0.61, 95%CI: 0.40-0.96, p 0.03). Conversely, a lower survival was associated with older age: patients aged 31-60 had 2-fold risk of death compared to those under 30 (HR 1.8, 95%CI 1.15-2.75, p <0.001); patients over 60 had a 3.2 times higher risk compared to under 30 (HR 3.2, 95%CI 2.35-4.45, p <0.001). Presence of trilineage cytopenia also predicted poorer survival (HR: 2.92, 95%CI: 2.25-3.79, p <0.001 versus single- or bi-lineage cytopenias). No clear association with survival was found regarding karyotype and presence of mutations at myeloid NGS panel (Table 1). Finally, via a 10-folds cross-validation, we built a multivariate logistic model to predict the probability of having an iBMF. A dataset composed of 139 patients (120 aBMF and 19 iBMF) was used to train and validate the model, while subjects with missing information were excluded from the analysis. The obtained classifier was tested on an independent test set, resulting in 96% (81-100) accuracy, with 26/27 patients correctly assigned to their true class, 100% sensitivity and 96% specificity and having area under the ROC curve (AUC) equal to 0.97. Factors showing an impact on the predictive model were as follows: higher Hb levels (OR 2.35, range 0.98-5.60, p 0.05), reticulocytes (OR 1.01, range 0.99-1.04, p 0.29), platelets (OR 1.05, range 1.01-1.09, p 0.005), and erythropoietin (OR 1.0, range 1.00-1.01, p 0.009) were associated with IBMF; whilst older age (OR 0.91, range 0.84-0.99, p 0.03), normal NGS myeloid panel (OR 0.09, range 0.01-1.52, p 0.09), and the presence of a PNH clone (OR 0.46, range 0.04-4.83, p 0.52) showed a negative association (Figure 1).
In conclusion, iBMF and aBMF differ in clinical presentation and risk of clonal evolution, and deserve specific treatments (IST in aBMF vs BMT and androgens in iBMF) and special considerations (i.e. choice of BMT donor and conditioning in iBMF). The proposed model includes few and routinely available variables and may be a practical tool to be implemented in the clinic, though it will require validation on an independent cohort to confirm its feasibility and prediction accuracy.
Disclosures
Gandhi:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SOBI: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Speakers Bureau. Fattizzo:Agios: Consultancy, Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Zenas Biopharma: Research Funding; Sobi: Speakers Bureau. Barcellini:Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kulasekararaj:Achillion: Consultancy; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Samsung: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Akari Therapeutics: Consultancy; BioCryst: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal